How Would You Classify Hydrogen
The Chemistry of Hydrogen
Hydrides
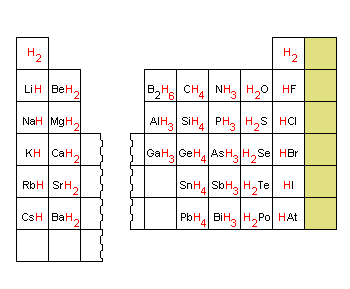
Hydrogen combines with every chemical element in the periodic tabular array except the nonmetals in Group VIIIA (He, Ne, Ar, Kr, Xe, and Rn). Although information technology is oftentimes stated that more compounds contain carbon than whatsoever other element, this is non necessarily true. Most carbon compounds also contain hydrogen, and hydrogen forms compounds with nigh all the other elements too. Compounds of hydrogen are oftentimes called hydrides, even though the name hydride literally describes compounds that comprise an H- ion. There is a regular trend in the formula of the hydrides across a row of the periodic table, as shown in the effigy below. This tendency is so regular that the combining ability, or valence, of an element was in one case defined as the number of hydrogen atoms bound to the chemical element in its hydride.
Hydrogen is the simply element that forms compounds in which the valence electrons are in the n = one shell. As a result, hydrogen can have iii oxidation states, corresponding to the H+ ion, a neutral H atom, and the H- ion.
| H+ | = | idue south 0 |
| H | = | 1s 1 |
| H- | = | 1southward two |
![]()
Hydrogen's Placement in the Periodic Table
Because hydrogen forms compounds with oxidation numbers of both +i and -ane, many periodic tables include this element in both Group IA (with Li, Na, M, Rb, Cs, and Fr) and Group VIIA (with F, Cl, Br, I, and At).
There are many reasons for including hydrogen among the elements in Group IA. It forms compounds (such as HCl and HNO3) that are analogs of brine metal compounds (such as NaCl and KNO3). Under weather condition of very high pressure, information technology has the properties of a metal. (It has been argued, for example, that any hydrogen nowadays at the center of the planet Jupiter is likely to exist a metallic solid.) Finally, hydrogen combines with a scattering of metals, such as scandium, titanium, chromium, nickel, or palladium, to class materials that acquit every bit if they were alloys of ii metals.
At that place are every bit valid arguments for placing hydrogen in Group VIIA. It forms compounds (such as NaH and CaH2) that are analogs of halogen compounds (such as NaF and CaCl2). Information technology as well combines with other nonmetals to form covalent compounds (such every bit H2O, CH4, and NH3), the fashion a nonmetal should. Finally, the element is a gas at room temperature and atmospheric pressure level, like other nonmetals (such as Oii and Ntwo).
It is hard to decide where hydrogen belongs in the periodic table because of the concrete properties of the element. The start ionization free energy of hydrogen (1312 kJ/mol), for example, is roughly halfway between the elements with the largest (2372 kJ/mol) and smallest (376 kJ/mol) ionization energies. Hydrogen also has an electronegativity (EN = 2.20) halfway between the extremes of the most electronegative (EN = 3.98) and least electronegative (EN = 0.7) elements. On the basis of electronegativity, it is tempting to classify hydrogen every bit a semimetal, as shown in the three-dimensional graph of the electronegativities of the chief-grouping elements shown beneath.
 |
| This three dimensional graph of the electronegativities of the chief-group elements helps u.s. empathise why it is difficult to allocate hydrogen as a metal or a nonmetal. |
Hydrogen is oxidized by elements that are more electronegative to form compounds in which it has an oxidation number of +1.

Hydrogen is reduced by elements that are less electronegative to form compounds in which its oxidation number is -1.

![]()
Properties and Formation of Hydrogen
At room temperature, hydrogen is a colorless, odorless gas with a density simply ane-fourteenth the density of air. Small quantities of H2 gas can be prepared in several ways.
ane. By reacting an active metal with h2o.
| 2 Na(s) | + | ii H2O(l) | | 2 Na+(aq) | + | two OH-(aq) | + | H two ( g ) |
2. By reacting a less active metallic with a strong acrid.
| Zn(s) | + | 2 HCl(aq) | | Zn2+(aq) | + | ii Cl-(aq) | + | H ii ( g ) |
3. By reacting an ionic metallic hydride with water.
| NaH(s) | + | H2O(l) | | Na+(aq) | + | OH-(aq) | + | H 2 ( g ) |
four. By decomposing water into its elements with an electrical current.
| electrolysis | |||||
| two H2O(l) | | 2 H 2 ( g ) | + | O2(one thousand) |
Use oxidation numbers to determine what is oxidized and what is reduced in the following reactions, which are used to gear up H2 gas.
(a) Mg(s) + ii HCl(aq) ![]() Mgii+(aq) + ii Cl-(aq) + H2(g)
Mgii+(aq) + ii Cl-(aq) + H2(g)
(b) Ca(southward) + two HiiO(50) ![]() Catwo+(aq) + 2 OH-(aq) + Htwo(thou)
Catwo+(aq) + 2 OH-(aq) + Htwo(thou)
Click hither to bank check your answer to Practice Problem 2
The covalent radius of a neutral hydrogen cantlet is 0.0371 nm, smaller than that of whatever other element. Because small atoms can come very close to each other, they tend to form strong covalent bonds. Every bit a result, the bond dissociation enthalpy for the H-H bail is relatively big (435 kJ/mol). Hii therefore tends to exist unreactive at room temperature. In the presence of a spark, however, a fraction of the H2 molecules dissociate to form hydrogen atoms that are highly reactive.
| spark | |||
| H2(g) | | 2 H(1000) |
The heat given off when these H atoms react with O2 is enough to catalyze the dissociation of additional H2 molecules. Mixtures of Htwo and O2 that are infinitely stable at room temperature therefore explode in the presence of a spark or flame.
![]()
How Would You Classify Hydrogen,
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/hydrogen.php
Posted by: smithbispecephe.blogspot.com


0 Response to "How Would You Classify Hydrogen"
Post a Comment